Corrosion control
To make the use of steel and other metals practical in construction and manufacturing, some corrosion-protection practices must be employed. Otherwise, the life of steel and other metals will be limited, reducing efficiency and escalating the cost of maintenance. There are several effective ways to stop corrosion:
1. Impressed current. By using suitable current-generating equipment and controls, it is possible to reproduce a current equal in strength to the corroding current, but flowing in the opposite direction. This type of protection is generally limited to pipelines, buried tanks, etc., and requires careful engineering and layout. Used improperly, an impressed current can promote corrosion.
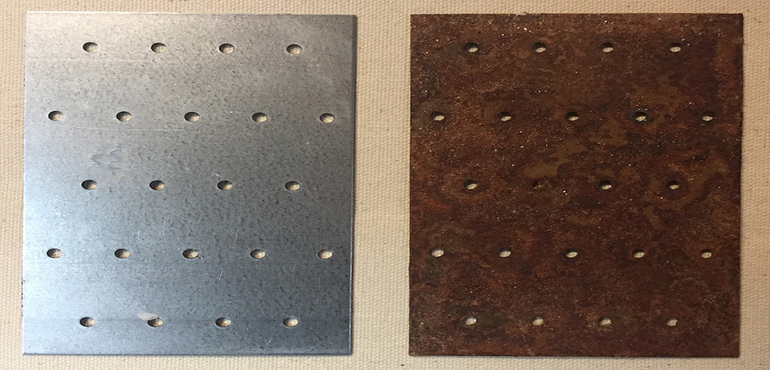
2. Sacrificial metals. Steel can be protected by adjacent placement to a dissimilar metal. For example, if zinc or magnesium is placed in direct contact with steel, it protects the steel from corrosion. Here, zinc and magnesium serve as sacrificial metals that not only protect the area of immediate contact, but also protect beyond the metal in each direction. Protection from rust by sacrificial metals is commonly used in several forms:
- Zinc or magnesium blocks are often used to protect ship hulls, water tank interiors and other submerged surfaces.
- Complete covering of the steel with the sacrificial metal is often done. Galvanized steel, for example, is steel covered with zinc. The zinc is sacrificial and will protect the base steel.
- Zinc-rich coatings may be applied to a steel surface to provide cathodic protection. Zinc-rich coatings consist of 85% to 95% zinc metal in a suitable binder. The zinc particles, deposited by painting, protect the steel.
3. Primers. Primers and finished coatings protect metal surfaces by providing a barrier between the steel and the corroding elements. They also prevent moisture from reaching the surface of the steel. A coating film protects underlying metal substrates in three ways:
- Coatings can slow the rate of diffusion of water and oxygen from the environment to the metal surface. This slows the corrosion process.
- The paint film can slow the rate of diffusion of corrosion products from the metal surface through the paint film. This also slows the corrosion process.
- The anti-corrosive pigments contained in quality primers change the surface properties of the base metal. The metal develops a high electrical resistance as a result. Different pigments accomplish this reaction in different ways. Primers absorb and tie up moisture so that it does not react with the steel.
How to choose a rust-proof coating
Considering the following criteria can reveal the most effective type of rust-proof coating needed for a specific project.
Quality of coat/applicatio – What level of anti-corrosive paint is needed? How important is it for paint to be fade-resistant and/or abrasion-resistant? How often do you expect to repaint? Is there an application preference: brush/roller or spray?
Aesthetics – What materials are going to be coated? How important is it for the paint coat to look attractive? Is color retention important?
Price – Typically, higher quality paint increases price. Are touch-up applications being considered when estimating the maintenance costs? What is the value of the paint selected? How often will it need to be recoated?